After twenty years of research on Deinococcus bacteria, renowned for their exceptional resistance to radiation and desiccation, BIAM celebrates an international recognition: the enzyme IrrE, an essential regulator of cellular survival under severe stress, has been included in the world reference book on peptidases. This chapter highlights the importance of fundamental research and opens new perspectives in microbiology, radiobiology, and biotechnology.
Twenty Years of Research on IrrE
At the invitation of editor Neil Rawlings, two BIAM researchers, Arjan de Groot and Laurence Blanchard, authored a chapter recounting twenty years of discoveries on the IrrE enzyme, from the characterization of the radiation-tolerant bacterium Deinococcus deserti in 2005—where it is present—to the full understanding of its mechanism of action, made possible by the identification of its substrate, the transcriptional repressor DdrO. This recognition symbolizes the culmination of a long-term effort involving also several PhD students and engineers, and highlights the functional uniqueness of IrrE, now considered the prototype of a new family of bacterial regulators involved in various stress responses.
A Key Enzyme for Extreme Resistance
Since its initial discovery in 2002 in Deinococcus radiodurans by an American team, and due to the work conducted at BIAM by these two scientists, IrrE has been recognized as an essential regulatory metallopeptidase for the cellular response to radiation and desiccation. The determination of its crystal structure in 2009, the discovery of its substrate, the DdrO repressor, in 2014, and the progressive elucidation of its molecular mechanism between 2017 and 2025 have positioned the IrrE/DdrO pair as a reference for a new family of bacterial regulators.
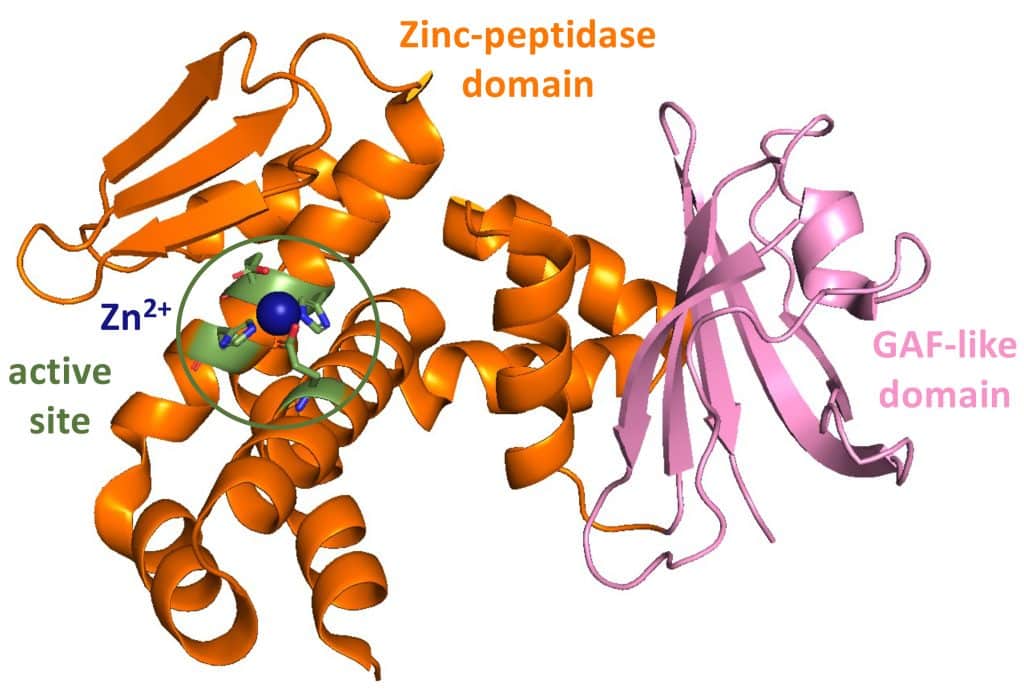
The mechanism of action is now well established: in response to radiation or desiccation that damages DNA, IrrE is activated and cleaves the DdrO repressor, leading to its inactivation. This step then triggers the expression of DNA repair genes, ensuring cell survival.
Bacterial Homologues of IrrE: New Avenues and Applications
Homologues of IrrE have been identified in numerous bacteria, whether environmental, pathogenic, or used in industry. Studying them could shed light on mechanisms of antibiotic tolerance, modulate the survival of pathogenic microorganisms, or optimize bacterial strains used in stress-prone bioproduction. This discovery highlights the importance of fundamental research in revealing key mechanisms of cellular survival and paves the way for new national and international collaborations involving CEA and CNRS with other partners.
An Inspiring Model, from Fundamental to Applied Science
IrrE perfectly illustrates how the study of a model organism can have a major scientific impact while inspiring innovative approaches for applied microbiology, radiobiology, and industrial biotechnology. This recognition, through its inclusion in an international reference book, opens new perspectives for research on bacterial stress responses and for the development of scientific collaborations and partnerships.
References
De Groot A.* & Blanchard L. (2025) IrrE peptidase (Deinococcussp.). Handbook of Proteolytic Enzymes. Metallopeptidases. 4th edition, Chapter 84 – pages 587-590.
DOI : 10.1016/B978-0-443-28849-4.00084-9
Bacterial radioresistance: Zinc, a precious metal!