Nanoscale Imaging
An international team of researchers has reached a technological milestone by managing to directly observe interactions between metals and living cells at the nanoscale1 . Thanks to a novel X-ray imaging technique, this advancement provides a new means to study relationships between metals and living systems—from how metallodrugs work to the behavior of nanoparticles in organisms.
It is now possible to observe iron in action within a living cell! Researchers from BIAM, in collaboration with the Max Planck Institute (Colloïds and interfaces, Potsdam, Germany) and the synchrotrons at Diamond Light Source in the UK and Soleil in Saclay (France), have developed an innovative approach to map metals in living microorganisms.
Why is mapping metals in living microorganisms important?
In living systems—specifically the dynamics of metal-biological interactions—metals play a crucial yet poorly understood role. For example, some bacteria can naturally produce magnetic nanoparticles composed of magnetite (Fe₃O₄), which they use like a compass to orient themselves in their environment. Many other organisms use metals to produce nanoscale minerals that can serve a biological function but also influence metal cycling in the environment. “Until now, imaging techniques did not allow these mechanisms to be observed in living systems: they lacked resolution or required artificial conditions, rendering these subtle dynamics invisible,” explains Daniel Chevrier, CNRS research scientist at BIAM and lead author of this new imaging approach.
An international collaboration to observe iron in native conditions
To overcome these technological barriers, the researchers pooled their expertise: BIAM for its knowledge in microbial biology, the Max Planck Institute for designing a microfluidic device, and the Diamond and Soleil synchrotrons for their state-of-the-art imaging techniques. Together, they successfully observed the formation of magnetite nanoparticles in live bacteria by measuring the amount of iron within individual cells over time.
A novel device to observe living cells
This breakthrough relies on two complementary innovations: First, the X-ray nanoprobe, an extremely fine beam produced by a synchrotron, capable of detecting metals at a very small scale. Second is a liquid cell, designed to keep bacteria alive throughout the observation process. Unlike conventional methods that often require samples to be frozen or dried, this microfluidic cell offers a stable environment close to natural conditions while withstanding the intensity of the X-rays. Thanks to this unique setup, researchers were able to monitor iron dynamics in magnetotactic bacteria over several hours.
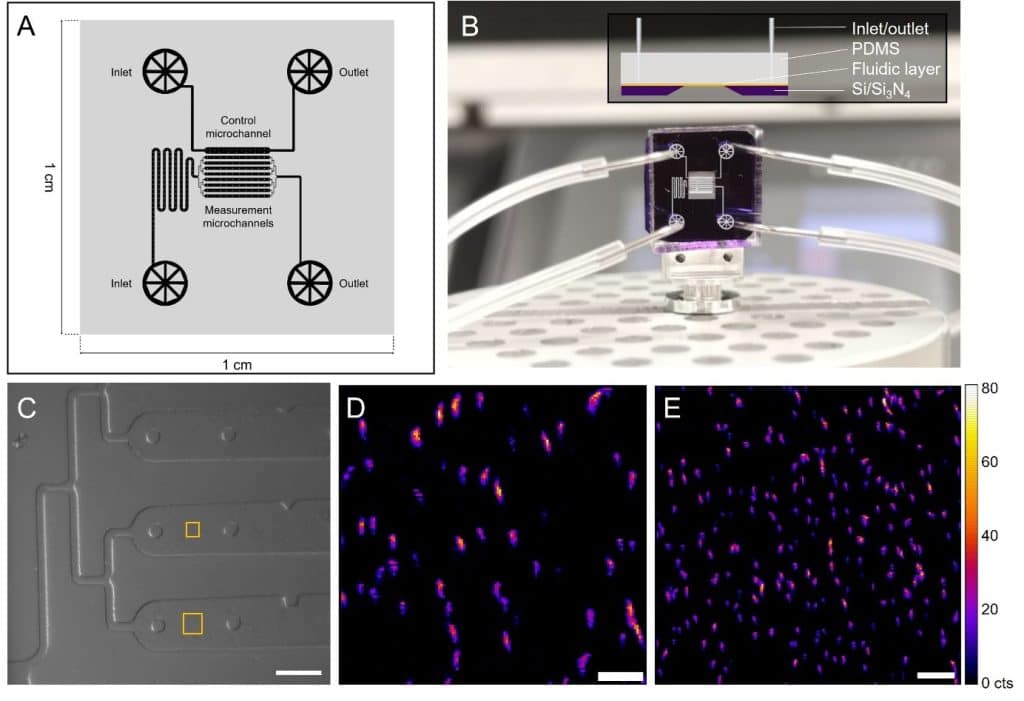
Custom microfluidic device for nano-XRF microscopy.
(A) Fabrication diagram of the microfluidic layer.
(B) Assembled device with connections and microchannel design (side view inset).
(C) Optical microscope image of the device installed on the scanning stage, with XRF analysis areas shown in yellow. (D, E) Maps of iron distribution (Fe Kα signal) in bacteria, obtained with a 150 nm (D) and 200 nm (E) scanning resolution. Color scale reflects XRF signal intensity as counts (cts). Scale bars: 100 μm (C), 3 μm (D), 5 μm (E).
Building on this success, the team is now turning to coccolithophores—microalgae capable of producing calcium carbonate shells—to better understand intracellular biomineralization through experiments in liquid environments.2
Why synchrotrons are central to this discovery
For a long time, the study of metals in living cells was limited by the lack of tools capable of observing them under near-natural conditions. Synchrotrons—large facilities that produce ultra-intense X-rays—are now opening a new frontier. They enable tracking, at the nanometric scale, of how metals interact with biological systems in a liquid environment compatible with cellular life.
“This is a qualitative leap: we can finally observe living cells without freezing them, while detecting trace amounts of metals,” the scientist enthuses. This unprecedented capability offers tremendous potential for life sciences, ecotoxicology, and medicine by providing data that reflect the real behavior of organisms. It allows for more accurate models, validation of hypotheses—and opens the door to sustainable innovations.
Promising applications for health and the environment
The experimental approach presented in this work could lead to major advances across several research fields. In particular, it may enhance understanding of the complex mechanisms behind metallodrugs (metal-based compounds used to treat certain diseases) and how cells absorb and break down nanoparticles.
In recent years, nanoparticles have attracted significant interest for their therapeutic potential, but their long-term impact on organisms and the environment remains unclear. This new imaging method may finally allow researchers to assess the state of these nanoparticles (e.g., composition, size) in complex media, and better understand their distribution and fate in living systems.
Additionally, the biomineralization of hard tissues—such as bones, teeth, or shells of certain marine species—could be studied in depth, thanks to the ability to observe the formation of these structures at the nanometric scale. These advancements promise new tools for researchers in biology, chemistry, and environmental sciences, with major implications for medicine and ecotoxicology.
In brief
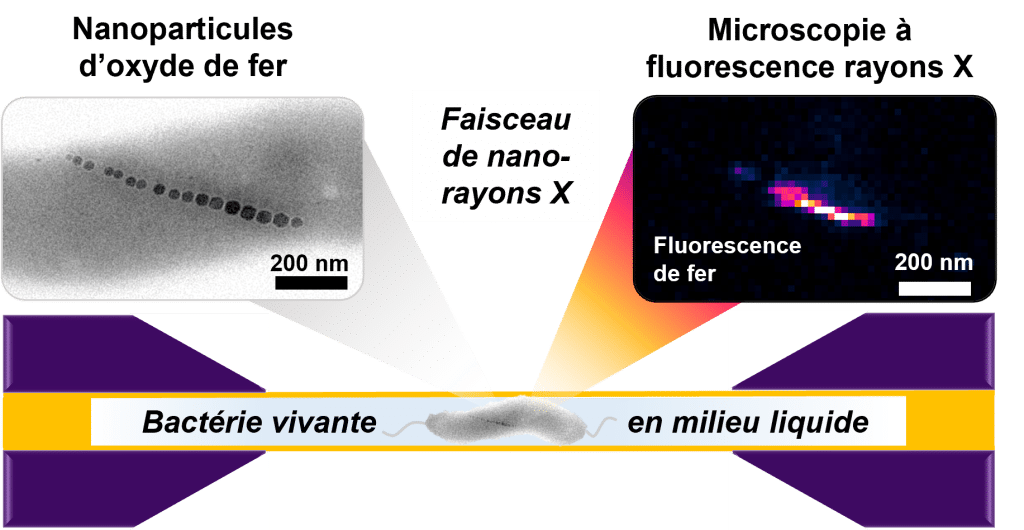
Schematic representation of X-ray fluorescence imaging of a live magnetotactic bacterium in a liquid cell.
The transmission electron microscopy image (top left) shows the iron oxide nanoparticle chain of a dried bacterium as a representative example. The X-ray fluorescence image (top right) shows the signal from iron in a living bacterium.
References
- M. Chevrier, E. Cerdá-Doñate, L. Gandarias, M. A. Gomez Gonzalez, S. Swaraj, P. E. D. SOTO RODRIGUEZ, A. Fraisse, T. Robinson and D. Faivre, Chem. Sci., 2025, Edge Article, DOI: 10.1039/D4SC08375J
- M. Chevrier, S. Gautam, A. Scheffel, Faraday Discuss., 2025 Advance Article, DOI: 10.1039/D5FD00021A