Understanding the functioning of PAF is essential because this photoenzyme represents a new opportunity for the sustainable production of biofuels from fatty acids naturally produced by living organisms. PAF is also very promising for the production of high value-added compounds for fine chemicals, cosmetics or pharmaceuticals. Finally, due to the fact that their reaction is triggered by light, photoenzymes give access to ultra-fast phenomena taking place during enzymatic reactions. Thus, PAF also represented a unique opportunity to understand in detail a chemical reaction taking place in living organisms.
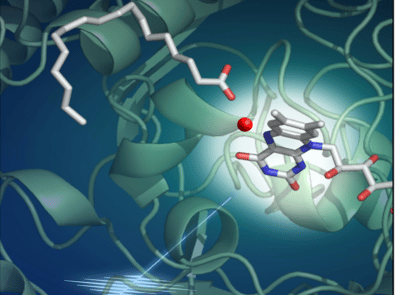
More precisely, in this work, the researchers show that when the PAF is illuminated and absorbs a photon, an electron is torn off in 300 picoseconds from the fatty acid produced by the algae. This fatty acid is then dissociated into hydrocarbon precursor and carbon dioxide (CO2). Most of the latter is then converted to bicarbonate (HCO3-) in 100 nanoseconds. This activity uses light, but does not prevent photosynthesis: the flavin molecule embedded in the PAF, which absorbs the photon, is bent. This conformation shifts the absorption spectrum of the molecule towards the red, so that it uses photons not exploited for the photosynthetic activity of the microalgae.
Plus précisément, dans ce travail, les chercheurs montrent que lorsque la FAP est éclairée et absorbe un photon, un électron est arraché en 300 picosecondes à l’acide gras produit par les algues. Cet acide gras est alors dissocié en précurseur d’hydrocarbure et en dioxyde de carbone (CO2). La majorité de ce dernier est ensuite transformée en bicarbonate (HCO3-) en 100 nanosecondes. Cette activité utilise de la lumière, mais n’empêche pas la photosynthèse : la molécule de flavine intégrée à la FAP, qui absorbe le photon, est courbée. Cette conformation déplace le spectre d’absorption de la molécule vers le rouge, de sorte qu’elle utilise des photons non exploités pour l’activité photosynthétique de la microalgue.
It is the combined interpretation of the results of various experimental and theoretical approaches by the international consortium that provides a detailed atomic-scale picture of the PAF at work. The multidisciplinary study combined work in bioengineering, optical and vibrational spectroscopy, static and kinetic crystallography performed with synchrotrons or an X-ray free electron laser, as well as quantum chemical calculations.
REFERENCES
D. Sorigué et al., “Mechanism and dynamics of fatty acid photodecarboxylase”, Science, 2021.