The living world has a multitude of organisms capable of synthesising mineral structures by incorporating materials present in their environment. Egg or mollusc shells, crustacean carapaces, coral, algae or phytoplankton exoskeletons are all illustrations of this biomineralisation, to which can be added the finer example of ‘magnetotactic’ bacteria, capable of moving along magnetic field lines.
“Unlike corals or crustaceans, these bacteria control biomineralisation by means of a specialised organelle, the magnetosome,” stresses Damien Faivre, a researcher at BIAM. “This organelle, located in the cytoplasm, at the heart of the cell, produces strings of iron oxide (magnetite) or iron sulphide (greigite) nanoparticles that probably enable the bacteria to orient themselves in relation to magnetic field lines”.

BIAM researchers and their partners have been interested in a particular strain (Desulfamplus magnetovallimortis BW-1) of magnetotactic bacteria that has the particularity of also biomineralising copper sulphide, as evidenced by the presence of nanoparticles in the space separating the cytoplasmic membrane and the outer membrane of the bacteria (periplasm).
The researchers compared strains BW-1 and RS-1 of the bacteria in the presence of zinc, nickel or cobalt ions. The result: BW-1 biomineralises but only copper, RS-1 does not biomineralise any of these elements.
These observations suggest that the copper sulphide biomineralisation observed in BW-1 is not a simple biologically-induced process, but rather a biologically-controlled process.
This is even strengthen by the fact that proteins potentially involved in the formation of copper sulphide nanoparticles were also identified.
The authors still need to understand the formation and function of the organelle that produces copper sulphide nanoparticles. In the long term, this discovery might open up new prospects for decontamination, but also techniques for harvesting fine-grained materials present in trace, which are currently impossible to collect.
Figure 1 : Some bacteria have the ability to form inorganic nanoparticles in a process called biomineralization. We show here a magnetotactic bacterium that precipitate an unknown type of intracellular copper-based nanoparticles in the periplasm. The biomineral has peculiar nanometric substructures, and a potential organic envelope surrounding the particles. Our proteomic data indicates a new type of biomineralization mechanism potentially involved in copper detoxification.
Figure 2 :
Transmission electron microscope image of a thin section of a BW-1 cell.
The copper sulfide particles, which appear dark after staining, are only located in the periplasmic space. The particles expand the periplasmic space, once their diameter becomes larger than the dimension available between the inner and the outer membranes.
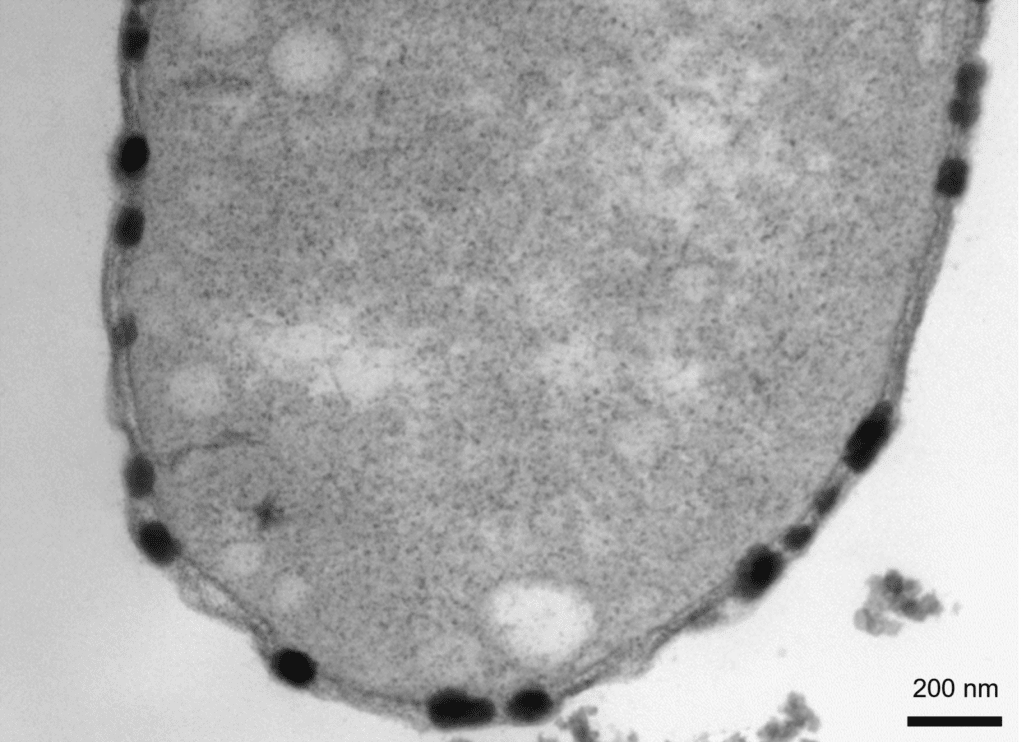
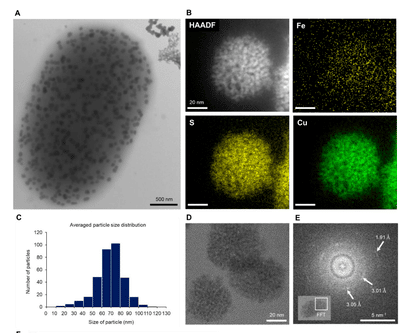
Figure 3 : Morphological and chemical analysis of copper sulfide nanoparticles produced by BW-1. (A) A BW-1 cell filled with intracellular nanoparticles, showing features distinct from extracellular precipitates, as imaged by TEM. (B) STEM HAADF image showing a single copper sulfide nanoparticle in a BW-1 cell and STEM EDS elemental maps of Fe, S, and Cu, respectively. (C) Particle size distribution (10 bacteria). (D) HRTEM image of copper sulfide particles composed of 1-2 nm substructures. (E) Fast Fourier transform of the boxed area in the inset, showing two faint rings and a few diffuse spots.
This work was carried out in collaboration with the Weizmann Institute of Science (Israel), the University of Pannonia (Hungary) and the CEA centre in Marcoule.
Periplasmic Bacterial Biomineralization of Copper Sulfide Nanoparticles
REFERENCES
Authors : Yeseul Park1, Zohar Eyal, Péter Pekker, Daniel M. Chevrier, Christopher T. Lefèvre, Pascal Arnoux, Jean Armengaud, Caroline L. Monteil, Assaf Gal , Mihály Pósfai, Damien Faivre
https://doi.org/10.1002/advs.202203444
Contact : Damien Faivre (BIAM)
All the scientific news: Fabrique de savoirs – Actualités scientifiques (cea.fr)