You are here : Home City of energies > HOME BIAM > Technological platforms > HélioBiotec – Microalgues > Supply of skills and equipment
Technical know-how and instruments

Responsable : Bertrand Légeret (IEHC CNRS)
HelioBiotec has all the necessary equipment to analyse microalgal lipids (membrane or storage glycerolipids) as well as other types of lipids (hydrocarbons, pigments, lipid polyesters and plant waxes).
Types of lipids analyzed:
- Glycerolipids: phospholipids, galactolipids, sulfolipids, mono-, di- and tri-acylglycerols, betaine lipids.
- Fatty acids: common and unusual species
Components of plant cuticular waxes: alkanes, primary and secondary alcohols, aldehydes, ketones, monoesters, etc. - Monomers of cutin and suberin polyesters: hydroxylated fatty acids, epoxidized fatty acids, dicarboxylic fatty acids, etc.
- Hydrocarbons: alkanes and alkenes
- Pigments: chlorophylls, carotenoids.
Instruments :
- Gas chromatography coupled to a mass spectrometer and/or a flame ionisation detector (GC-MS/FID): 1 GC-MS (TRACE-DSQ Thermo), 1 GC-MS/FID with automatic sample preparation (Agilent), 1 GC-MS/FID (Agilent) with thermodesorber (Gerstel).
- High performance thin layer chromatography (HPTLC): 1 Camag TLC autosampler 4
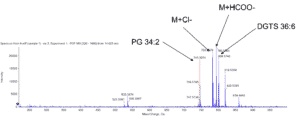
- Liquid chromatography/mass spectrometry (UPLC-MS/MS): 1 QTOF (Triple TOF 5600 AB SCIEX), 1 Orbitrap (Q Exactive Thermo).
Glycerolipid analysis:
- Different types of analysis
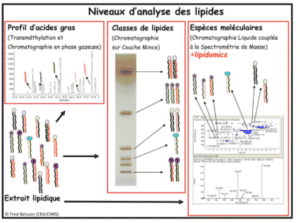
- Profil d’acides gras (par GC-MS/FID)
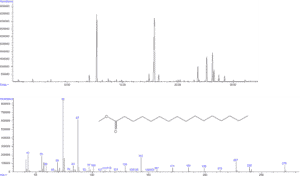
© B. Légeret / CEA
- Lipid classes (by HPTLC)
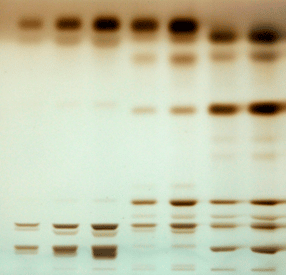
© C. Cuiné / CEA
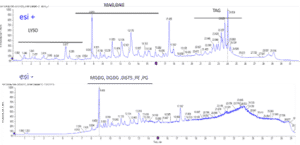
- Lipid molecules spicies (by UPLC-MS/MS)
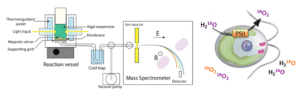
The HelioBiotec platform is equipped with two mass spectrometers dedicated to the analysis of gases of biological interest and more specifically oxygen, hydrogen and carbon dioxide
Mass spectrometry enables the composition of the atmosphere (gaseous or dissolved) to be monitored in real time in contact with a biological sample: living cells (microalgae, bacteria, isolated cells, etc.) or an enzyme extract. This technique is used to characterise photosynthetic oxygen or carbon dioxide exchange or hydrogen photo-production processes in microalgae or cyanobacteria. It allows the monitoring of gaseous species marked by stable isotopes to determine unidirectional flows (oxygen uptake by light).
The dissolved gases contained in the analysis cell (useful volume of the order of 2 mL) diffuse through a Teflon membrane, then transit via a vacuum line to the mass spectrometer’s ion source. This technique allows the real-time analysis of gas exchange of different types of biological materials (microalgae, bacteria, enzymes, etc.). The suspension can be illuminated either with white light supplied by fibre optics or with green light supplied by 3 LEDs that distribute the illumination uniformly. The fleet consists of a PrismaPlus quadrupole analyser (Pfeiffer Vacuum) and a Prima dB magnetic sector analyser (Thermo Scientific).
In order to select the strains of interest, the laboratory has developed two screening methods. The first discriminates on lipid composition, the second on the photosynthetic activity of the organism.
- Screening of a mutant library for TAG content

- Screening of a mutant library on fatty acid composition
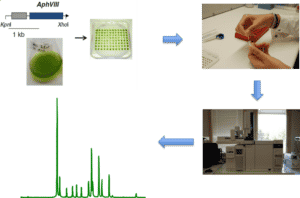
- Screening of a mutant library on chlorophyll fluorescence
Chlorophyll fluorescence was measured in vivo using a FluorCam FC 800 video imaging device (Photon Systems Instruments).

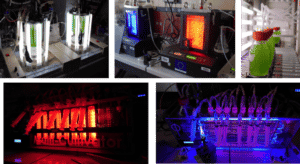
This closed culture system allows for very fine control of the various parameters that regulate the growth of microalgae, temperature, light intensity, pH, agitation speed and carbon dioxide flow.
We have four autoclavable bioreactors with a useful volume of 1 litre (BIOSTAT® Aplus, Sartorius Stedim Biotech) for the culture of microalgae. This device allows the control of parameters such as temperature, pH, stirring speed, culture gas flow and light intensity.
An overflow probe and a peristaltic pump for the addition of medium allow continuous cultivation. The lighting system in the laboratory allows the lighting to be regulated between 30 and 1500 µmol photon/m2/s (PAR). These bioreactors are controlled from a computer with the PC Panel µDCU software.

To study, in vivo, the parameters determining the efficiency of solar energy conversion by photosynthesis, the platform has non-invasive optical spectroscopy techniques
Dual Pam: This device, based on modulated analysis light, makes it possible to follow the fluorescence of the chlorophyll of photosystem II and the absorption of the reaction centre of photosystem I in real time.
In particular, it allows the estimation of the light energy conversion efficiencies of these two photosystems.
JTS10 (Joliot-type spectrophotometer): This spectrometer measures changes in the absorption of pigments and electron carriers in the photosynthetic chain. As the measurement is carried out using flashes of the order of a microsecond, it allows a fine kinetic resolution of the various electron transfer events contributing to photosynthesis.
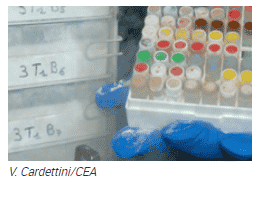
Strains stored at EBM
A collection of nearly 500 strains of microalgae and cyanobacteria, both wild and transformed, is stored in the cryobank of the EBM team, which hosts the Heliobiotec platform:
Chlamydomonas reinhardtii insertion mutants created in the laboratory
C. reinhardtii strains expressing genes of interest up or down
Synechocystis PCC6803 mutant strains
Various microalgal species from collections around the world (CCAP, UTEX, SAG, Duke Center, etc.)
Conservation of strains
Microalgae are preserved by freezing them at a temperature below -180°C in a cryoconservator in liquid nitrogen. This method of conservation was chosen to avoid the genetic drift that can occur following too many transplants on agar.
Strain management
The LabCollector software is used to manage the strains at the EBM.
This software allows us, among other things, to manage two important steps in the conservation of strains:
Traceability of operations
The planning of periodic viability tests
At the time of the viability controls, non-contamination tests are also carried out.
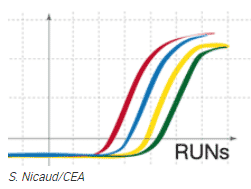
Objective: To compare the evolution of different genes of interest for the production of biofuels or biosourced molecules for green chemistry, cosmetics, pharmaceuticals and nutraceuticals.
The HelioBiotec platform has a LightCycler 480 (Roche Diagnostics) which allows us to carry out gene quantification in real time.
This is a fusion of two technologies:
PCR: polymerase chain reaction allowing the amplification of DNA
Quantification (relative or absolute) of the fluorescent signal produced
The aim is to compare the evolution of different genes of interest. This comparison is normalised by control genes (stable under all conditions). The analyses can be performed in 96 or 384 well plates.
This device also allows genotyping to be carried out using the HRM (High Resolution Melting) module. This technique can be used, for example, to separate 2 variant populations for a single base (nucleic acid) in a given gene. It combines the same technologies as above but with better resolution.
Responsible & contact
Key words
alkanes and alkenes; glycerolipids; phospholipids; hydrocarbons; pigments; lipid polyesters; vegetable waxes; dicarboxylic fatty acids; lipid droplets