You are here : Home City of energies > HOME BIAM > Search > PPV > PPV Research Themes
Research topics
Analysis of physiological functions of thiol reductases, enzymes controlling the redox status of proteins, in plant responses to environment constraints
Reactive oxygen species lead to post-translational modifications that can provoke changes in activity and/or conformation of proteins. These modifications are generally irreversible except in the case of the two sulfur-containing residues, cysteine and methionine. Indeed, the redox status of these amino acids is regulated by a superfamily of enzymes termed thiol reductases and including thioredoxins, glutaredoxins, peroxiredoxins and methionine sulfoxide reductases.
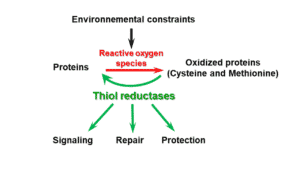
Figure 1 : Functions of thiol reductases in plant responses to environmental constraints
These enzymes, by controlling the redox status of target proteins, play decisive roles in protective and repair mechanisms of photosynthetic structures upon environmental constraints, as shown for the thioredoxin CDSP32 (Chloroplastic Drought-induced Stress Protein of 32 kDa) and methionine sulfoxide reductases (Photo 1).

We also showed the involvement of glutaredoxins GRXS14 and GRXS17 in light-dependent signal transduction pathways controlling development (Photo 2).

Photo 2 : Development of Arabidopsis plants; Plants WT (left) or déficient in glutaredoxin GRXS17 (right) under long photoperiod (16 h). © CEA/BIAM P. Rey
Our current research aims at deciphering the roles of thiol reductases in the functioning of stomata, which are leaf pores surrounded by two guard cells that have the capacity to modulate their volume as a function of environmental conditions. Stomata allow gas and water exchange at the leaf surface, and thus condition photosynthetic activity and plant growth. By performing a proteomic approach, we showed that many thiol reductases are abundant in guard cells. Further, we observed that in optimal growth conditions the stomatal conductance as well as the leaf temperature are altered in Arabidopsis lines deficient for the expression of genes coding for plastidial peroxiredoxins and glutaredoxins (Photo 3). These mutants also display increased sensitivity to abscisic acid and hydrogen peroxide, which are critical hormonal and redox messengers promoting stomatal closure.
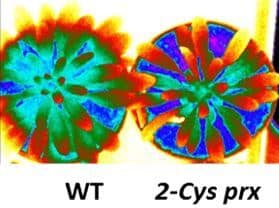
Taken collectively, these data unveil an essential role of thiol-based switch mechanisms in the signaling network controlling stomatal movements.
Our objective is to further delineate the physiological functions of thiol reductases and of their partners/targets within the signaling networks regulating the activity of photosynthesis and the functioning of stomata in relation to environmental stimuli.
Contact : Pascal REY
Role of plastidial lipocalin in the protection of photosynthetic apparatus
Figure 1 : Lipocaline © CEA/BIAM D. Rumeau
Lipocalins constitute an evolutionary conserved family of small proteins widely distributed in nature whose common feature is their ability to bind small hydrophobic molecules. Related to their ligands, lipocalins can fulfill a wide variety of functions such as transport of small molecules, regulation of developmental processes, signal transduction and response to stress.
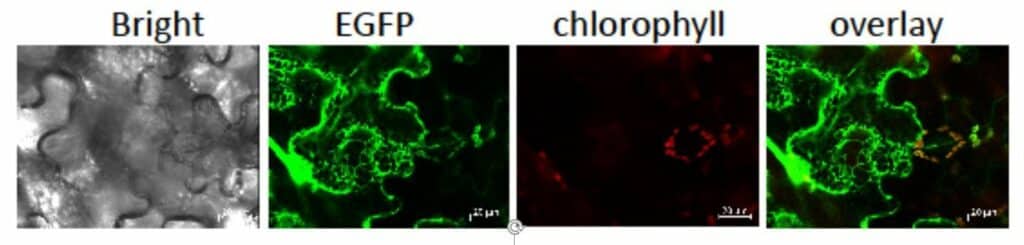
Photo 1 : Localization of LCNP. Nicotiana benthamiana leaves were agroinfiltrated for the transient expression of APO-EGFP. Confocal scanning microscopy observations were performed at 6 days post-infiltration
Because of their possible involvement in various diseases including lipid disorders, neurodegenerative diseases and cancer, human and animal lipocalins are extensively studied.
Two different lipocalins, which were classified as temperature-induced lipocalin (TIL) and chloroplastic lipocalin (LCNP), have been identified in plants.
Both lipocalins are involved in plant abiotic stress tolerance, however the molecular mechanisms underlying this function remain to be elucidated.
Our research work focuses on the plastidial lipocalin (Photo 1). LCNP is induced in response to various abiotic stresses including high light, dehydration and low temperature. It contributes to protection against oxidative damage promoted by harmful conditions (Photo 2) by preventing accumulation of hydroxy fatty acids and lipid peroxidation.

Moreover, it has been demonstrated that the plastid lipocalin contributes to qH, a non-photochemical quenching component, which operates under stress conditions such as cold and high light, and appears to be photoprotective (Figure 2, collaboration M Havaux (SAVE, BIAM) and A Malnoë (Umea University, Sweden).
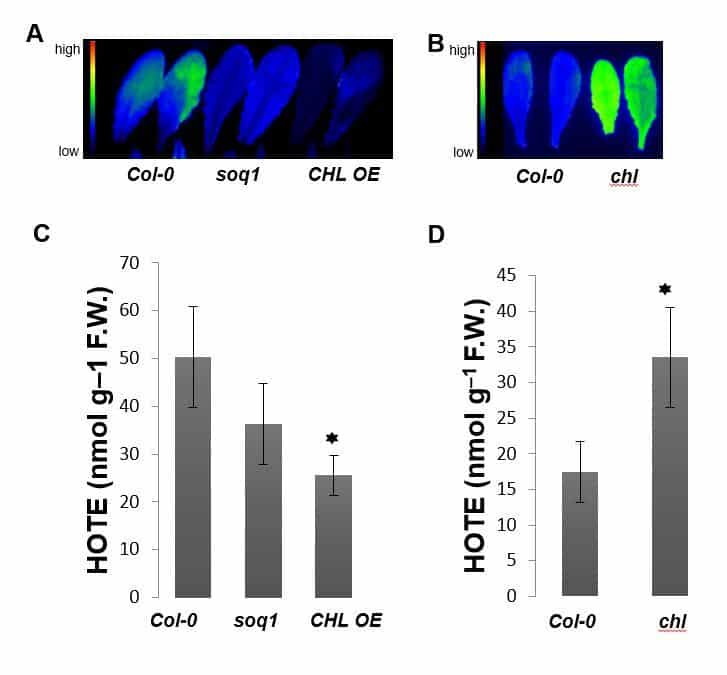
We are in the midst of identifying LCNP ligands, an indispensable prerequisite for understanding the mechanism underlying the LCNP protective function.
In guard cells, the activity of a lipoxygenase contributes to alkylation of a protein kinase and thus causes stomatal closure induced by biotic stresses
In our team, we are also exploring a signaling pathway that contributes to innate immunity of plants. This route involves oxidative stress and in particular, a lipoxygenase present in the guard cells.
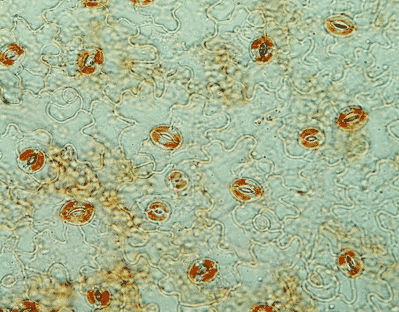
Photo 1 : Arabidopsis leaf epidermis and stomata. Neutral red staining. © CEA/BIAM J-L. Montillet
Since guard cells (Photo 1) control a significant part of gas exchanges required for photosynthesis, another objective of our scientific project is to understand how physical and biological threats can interfere with this important function in plants. In Arabidopsis, inoculation of the plant leaves with the bacterium Pseudomonas syringae is followed by activation of lipoxygenase 1 (LOX1), present in guard cells. This enzyme is involved in the production of reactive oxylipins that trigger stomatal closure. We are studying this original signaling pathway of the guard cell that prevents penetration of the pathogen and colonization of the plant.
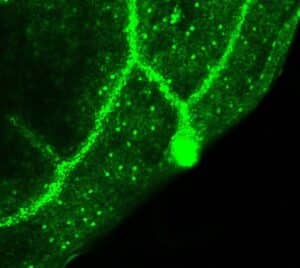
Photo 2 : Localization of eGFP fluorescence in plants transformed with RRPK1 promoter::eGFP reporter. Fluorescence is localized in stomata, conducting tissues and hydathodes. © CEA/BIAM D. Rumeau
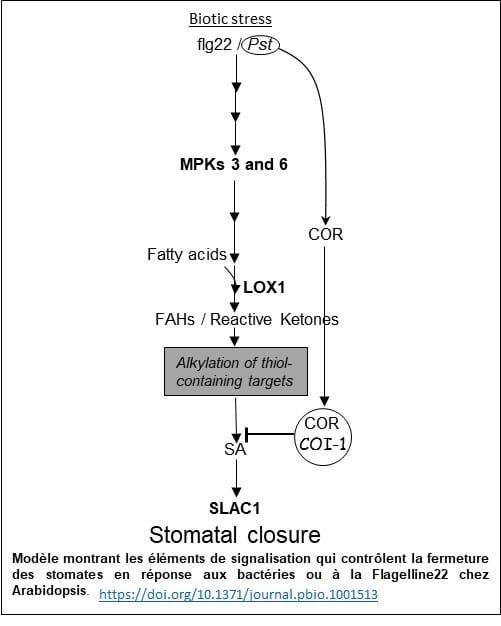
A “click chemistry” approach allowed us to identify a serine-threonine protein kinase that is localized in stomata (Photo 2) and plays a crucial role in this signaling pathway. The mechanism by which this protein kinase operates in this defence phenomenon is currently being elucidated (Model).
Contact : Jean-Luc MONTILLET
Team manager
Pascal Rey
Contact the team
Key words
Higher plants, environmental constrains, stress responses, chloroplast, photosynthesis, proteins, lipocalin, thiol reductases, oxidoreduction, signalization, stomata.